Abstract
Background: Dynamic remodelling of gene regulatory networks (GRN) guides cell state changes during differentiation to establish and maintain cell identity and gene expression. We previously described combinatorial binding of 7 transcription factors (TF (heptad: FLI1, ERG, GATA2, RUNX1, TAL1, LYL1 and LMO2)) which form an autoregulatory network in human hematopoietic stem and progenitor cells (HSPC: Beck et al Blood 2013, Thoms et al Blood 2021). Here we mapped genome-wide binding of TFs and chromatin regulators and explored their roles in establishing and maintaining cell type specific GRNs across human hematopoietic stem cell (HSC) differentiation.
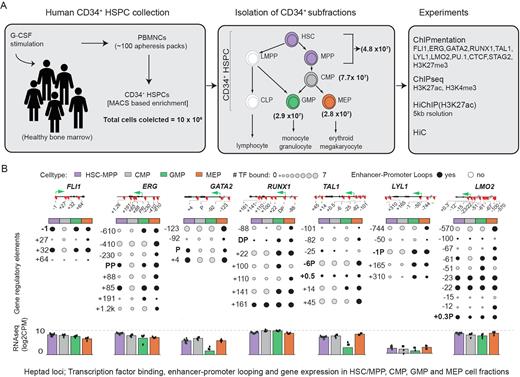
Methods: We flow sorted cryopreserved CD34+ LIN- cells collected by apheresis into HSCs (CD38loCD45RA-), common myeloid progenitors (CMP: CD38hiCD45RA-CD123+), granulocyte monocyte progenitors (GMP: CD38hiCD45RA+CD123+) and megakaryocyte erythroid progenitors (MEP: CD38hiCD45RA-CD123-). Active regulatory regions (Fig A) were identified by a) chromatin immunoprecipitation (ChIP)/ChIPmentation for genome wide identification of active (H3K27ac, H3K4me3) and inactive (H3K27me3) histone marks, and TF and co-factor (heptad, PU.1, STAG2, CTCF) binding, b) HiC to broadly classify high order 3D genome structures and c) H3K27ac HiChIP at 5kb.
Results: Combinatorial binding of heptad TFs in all cell types was confirmed, with the most overrepresented pattern being co-binding of all 7 factors. However, certain multifactor combinations were identified in restricted cell types or associated with lineage specific gene promoters. For example, the master erythroid TF GATA1 showed combinatorial binding in CMPs and MEPs, while the myeloid TF MPO showed combinatorial binding in CMPs and GMPs.
Chromatin looping was highly variable across the four cell types, although high order genome architecture was conserved. We first focussed on HiChIP interactions involving promoters of the heptad genes and observed chromatin looping patterns consistent with an interconnected circuit. Promoter looping patterns were dynamic across cell types, and we found multiple distal regions, each with specific patterns of TF binding, connected to each heptad promoter (Fig B). Many of the distal regions were previously undescribed and may constitute novel regulators of heptad genes.
To further understand the role of heptad TFs in regulating cell type specific gene expression, we used our HiChIP data to link promoters to candidate distal regulatory regions and focussed on promoter-regulator pairs with differential heptad binding across the cell types. In general, differential binding was accompanied by differential gene expression. Genes with high heptad binding in MEPs were enriched for pathways related to erythroid cells (e.g., ferroptosis signalling) while genes with high heptad binding in GMPs were enriched for pathways related to myeloid cells (e.g., TREM1 signalling). We then focussed on promoter-regulator pairs corresponding to genes expressed in mature erythroid and myeloid cells and observed cell specific patterns of TF binding. At erythroid genes we saw increasing GATA2/TAL1/LYL1/LMO2 binding and decreasing ERG binding along the HSC to MEP trajectory, while at myeloid genes we saw high GATA2/LYL1/LMO2 binding and low TAL1 binding along the HSC to GMP trajectory.
To conduct an unbiased genome wide analysis of HSPC GRNs we used our data to annotate and cluster the 85,100 accessible chromatin regions present in HSPCs (Corces et al Nature Genetics 2016). Accessible regions clustered broadly as promoters, regulatory regions, or CTCF-bound. Regions with high heptad binding in either MEPs or GMPs were located in different subclusters, suggesting dynamic reorganisation of heptad TFs across the genome as HSCs proceed along differentiation trajectories. Taken together our data strongly supports a mechanistic link between differential heptad TF binding and cell type specific expression patterns and provides insight into how expression of multiple genes is coordinated during cell state transitions.
Conclusion: This study provides a comprehensive characterisation of the gene regulatory landscape in rare subpopulations of human HSPCs and will be a key resource for understanding adult hematopoiesis. Our work provides a framework for analysing aberrant regulatory networks in leukemic cells and leveraging these to devise novel therapeutic strategies.
Disclosures
Gandhi:Janssen: Research Funding; Beigene: Research Funding. Pimanda:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.